Old Browser
Your account has been put on hold due to inactivity. To re-activate, check your account information and make all necessary updates.
Looks like you're visiting us from {{countryName}}.
Would you like to remain on the current country site or be redirected to one based on your location?
Your account has been put on hold due to inactivity. To re-activate, check your account information and make all necessary updates.
BD IMag™ Human Dendritic Cell Enrichment Set - DM
(RUO)



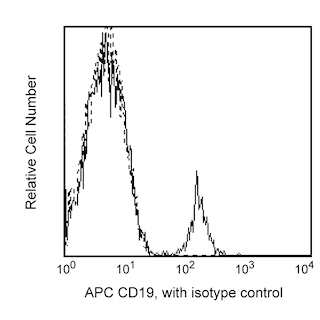
Enrichment of dendritic cells from human blood. PBMC were labeled with the BD IMag™ Human dendritic cell Enrichment Set - DM (Cat. No. 558420) and separated on the BD IMag™ Cell Separation Magnet(Cat. No. 552311) according to the accompanying protocol. To demonstrate the efficiency of the enrichment, cells were stained with a lineage cocktail consisting of APC Mouse Anti-Human CD3 (Cat. No. 555335), CD19 (Cat. No. 555415), CD14 (Cat. No. 555399), and CD56 (Cat. No. 555518) and a dendritic cell cocktail consisting of PE Mouse Anti-Human CD11c (Cat. No. 555392), CD123 (Cat. No. 555644), CD16 (Cat. No. 555407) and CD34 (Cat. No. 550761). Dead cells were excluded by staining with 7-Amino-actinomycin D (7-AAD) (Cat. No. 559925). Flow cytometry was performed on a BD FACSCalibur™ flow cytometry system. Please refer to the Enrichment Flow Chart to identify the cell populations represented in this figure. The percentage of dendritic cells is indicated in the upper-left corner of each panel. Panel A shows unseparated PBMC. Panel B shows the twice-enriched fraction after three 6-minute magnetic separations with an additional 10-minute separation. Panel C shows the positive fraction.


BD IMag™ Human Dendritic Cell Enrichment Set - DM

BD IMag™ Human Dendritic Cell Enrichment Set - DM
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Description
The BD IMag™ Human Dendritic Cell Enrichment Set - DM is used for the negative selection of dendritic cells (DC) from peripheral blood. The Human Dendritic Cell Enrichment Cocktail contains biotinylated monoclonal antibodies that recognize antigens expressed on erythrocytes, platelets, and peripheral leukocytes that are not DC. The BD IMag™ Streptavidin Particles Plus - DM are magnetic nanoparticles that have streptavidin covalently conjugated to their surfaces. With these two components, the BD IMag™ Human Dendritic Cell Enrichment Set - DM avoids the inadvertent activation of the enriched DC by using reagents that do not directly bind to those DC. This Enrichment Set has been optimized for use with the BD IMag™ Cell Separation Magnet, and it contains sufficient reagents to label 10e9 peripheral blood mononuclear cells (PBMC).
The Human Dendritic Cell Enrichment Cocktail, 5.0 mL, is comprised of the following biotin-conjugated monoclonal antibodies: Biotin Mouse Anti-human CD3, clone UCHT1
Biotin Mouse Anti-human CD14, clone M5E2
Biotin Mouse Anti-human CD19, clone HIB19
Biotin Mouse Anti-human CD41a, clone HIP8
Biotin Mouse Anti-human CD56, clone B159
Biotin Mouse Anti-human CD66b, clone G10F5
Biotin Mouse Anti-human CD235a (Glycophorin A), clone GA-R2 (HIR2)
Biotin Mouse Anti-human IgE, clone G7-26
Preparation And Storage
Recommended Assay Procedures
The detailed Magnetic Labeling and Enrichment Protocol follows. In summary, the Human Dendritic Cell Enrichment Cocktail simultaneously stains erythrocytes, platelets, and most leukocytes except the DC. After washing away excess antibody, BD IMag™ Streptavidin Particles Plus - DM are added to the cell suspension and bind the cells bearing the biotinylated antibodies. The tube containing this labeled cell suspension is then placed within the magnetic field of the BD IMag™ Cell Separation Magnet (Cat. No. 552311). Negative selection is then performed to enrich for the unlabeled DC. Labeled cells migrate toward the magnet (positive fraction), leaving the unlabeled cells in suspension so they can be drawn off and retained (enriched fraction). The negative selection is repeated twice to increase the yield of the enriched fraction. If greater purity is required, negative selection may be performed on the enriched fraction. For clarification of the procedure, the magnetic separation steps are diagrammed in the Enrichment Flow Chart. The positive and enriched fractions can be evaluated in downstream applications such as flow cytometry and tissue culture. The biotinylated antibodies in the Human Dendritic Cell Enrichment Cocktail have been optimized and pre-diluted to provide maximum efficiency for the enrichment of DC from PBMC.
MAGNETIC LABELING AND ENRICHMENT PROTOCOL
1. Prepare 1X BD IMag™ buffer: Dilute BD IMag™ Buffer (10X) (Cat. No. 552362) 1:10 with sterile distilled water or prepare Phosphate Buffered Saline (PBS) supplemented with 0.5% BSA, 2 mM EDTA, and 0.1% sodium azide.
2. Prepare PBMC from anti-coagulated human blood, preferably by density gradient centrifugation using Ficoll-Paque™.
3. Remove clumps of cells and/or debris by passing the suspension through a 70-μm nylon cell strainer. Count the cells, and resuspend them in 1X BD IMag™ buffer at a concentration of 50 x 10e6 cells/ml.
4. Add the Human Den dritic Cell Enrichment Cocktail at 5 μl per 1 x 10e6 cells, and incubate at room temperature for 15 minutes.†
5. Wash the labeled cells with a 10X excess volume of 1X BD IMag™ buffer, centrifuge at 300 × g for 7 minutes, and carefully aspirate ALL the supernatant.
6. Vortex the BD IMag™ Streptavidin Particles Plus - DM thoroughly, and resuspend the cell pellet in 7.5 μl of particles per 1 x 10e6 cells.
Please note that this volume of IMag Streptavidin Particles is higher than that used in most BD IMag enrichment sets and that the Human Dendritic Cell Enrichment Set - DM contains a greater total volume of these particles to account for this difference.
7. MIX THOROUGHLY . Incubate at room temperature for 30 minutes.†
8. Bring the labeling volume up to a concentration of 20 to 80 x 10e6 cells/ml with 1X BD IMag™ buffer.
9. Transfer the labeled cells to a 12 x 75 mm round-bottom test tube, maximum volume added not to exceed 1.0 ml. Place this positive-fraction tube on the Cell Separation Magnet (horizontal position) for 6 to 8 minutes.
• For greater volume, divide the cells into multiple 12 X 75 mm round-bottom test tubes or transfer the cells to a 17 x 100 mm round-bottom test tube, maximum volume added not to exceed 3.0 ml. Place this positive-fraction tube on the Cell Separation Magnet (vertical position) for 8 minutes.
10. With the tube on the Cell Separation Magnet and using a sterile glass Pasteur pipette, carefully aspirate the supernatant (enriched fraction) and place in a new sterile tube.
11. Remove the positive-fraction tube from the Cell Separation Magnet, and add 1X BD IMag™ buffer to the same volume as in Step 8. Resuspend the positive fraction well by pipetting up and down 10 to 15 times (avoid creating bubbles), and place the tube back on the Cell Separation Magnet for 6 to 8 minutes.
• For 17 x 100 mm tube: Place on the Cell Separation Magnet for 8 minutes.
12. Using a new sterile Pasteur pipette, carefully aspirate the supernatant and combine with the enriched fraction from Step 10 above.
13. Repeat Steps 11 and 12. The combined enriched fraction contains dendritic cells with no bound antibodies or magnetic particles.
14. To increase the purity of the combined enriched fraction, place the tube containing the combined enriched fraction on the Cell Separation Magnet for another 10 minutes.
• For 17 x 100 mm tube: Place on the Cell Separation Magnet for 10 minutes.
15. Carefully aspirate the supernatant and place in a new sterile tube. This is the twice-enriched fraction. The cells are ready to be processed for downstream applications.
16. The positive-fraction cells remaining in the original tube can be resuspended in an appropriate buffer or culture medium for downstream applications, including flow cytometry, if desired.
17. Samples of the total cell suspension and the positive and enriched fractions should be analyzed by flow cytometry to evaluate the efficiency of the cell-separation procedure.
NOTES:
• Draw the blood into a tube containing EDTA
• Remove the platelet rich plasma by centrifuging once at 220-240 × g.
• Wash 2-3 times in PBS after the density gradient separation.
• After the final wash, resuspend the cells at a relatively high concentration in 1X BD IMag™ buffer and proceed to step 3.
† Avoid nonspecific labeling by working quickly and adhering to recommended incubation times.
Product Notices
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- BD IMag™ particles are prepared from carboxy-functionalized magnetic particles which are manufactured by Skold Technology and are licensed under US patent number 7,169,618.
- Ficoll-Paque is a trademark of Amersham Biosciences Limited.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
Companion Products





| Description | |
|---|---|
| Streptavidin Particles Plus – DM | N/A |
| Human Dendritic Cell Enrichment Cocktail | N/A |
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.