Fluorescence-Activated Cell Sorting and BD Biosciences: A Shared Past, Intertwined Journey, and Boundless Future
January 10, 2024
Fluorescence-activated cell sorting, or FACS, has revolutionized how cells can be identified, characterized and sorted using flow cytometry. It propelled scientists from the tedium of counting cells one by one in a dark room to the joy of rapidly obtaining deep scientific insights with the specificity and sensitivity required to turn that knowledge into meaningful clinical practices. BD Biosciences has always been there, right from incorporation of this technology into a viable commercial instrument to seeing it flourish through its wide adaptation in routine scientific experiments and clinical practices. As we celebrate the 50th anniversary of the commercialization of the first-ever FACS instrument by BD Biosciences, we reflect on the genesis of FACS as a technology, evolution of flow cytometry as a technique and the enormous scientific progress FACS has facilitated in the modern era.
Like many groundbreaking discoveries, cell sorting had a humble beginning. Its roots lay in an in-house modification of a Coulter counter, originally used to count cells, into a sort of cell sorter by Mack Fulwyler at the Los Alamos Laboratory.1 His setup used the same technology to separate different types of cells when they passed through a liquid stream based on differences in electrical resistance, which were proportional to differences in their volume. He modified it to break the liquid stream into individual droplets and sorted the cells based on their electrical charges.
Leonard “Len” Herzenberg, a geneticist at Stanford and a genius with an indomitable spirit, tweaked this modest cell sorter developed by Mack Fulwyler and converted it into what would become FACS, the technology that would transform biomedicine, immunology research, and disease diagnosis and monitoring in the years to come. Immunology researchers at the time, including Len, were using immunofluorescence to painstakingly count fluorescently stained cells under a microscope in a dark room. Wanting to combine immunofluorescence with flow cytometry to identify and sort cells, Len utilized Fulwyler’s cell sorting technology, merged it with the ink-jet printer technique developed by Richard Sweet at Stanford and built a cell sorter capable of identifying and sorting cells based on cell surface markers tagged with fluorescently stained antibodies.2 Instead of electrostatic charges, he used a light source that allowed the detection of mouse splenocytes immunized with Chinese hamster ovary (CHO) cells. He used fluorescein diacetate dye for labeling biomarkers and, in 1969, successfully sorted antibody-producing splenocytes from CHO cells based on differential fluorescence activity using his FACS (Fluorescence Activated Cell Sorter).2 From that modest cell sorter to the present-day sophisticated cell analyzers, FACS technology, and its enablement of scientific discoveries, has evolved in leaps and bounds.
Flow cytometry technology evolution
How did flow cytometry instrumentation evolve from an ordinary-looking contraption with just a breadboard, oscilloscope screen and polaroid cameras to modern-day sophisticated flow cytometers? How did it move from research labs to clinic and even to the International Space Station?3 This evolution required collaboration between extraordinary scientific minds, like that of Len Herzenberg at Stanford, and the commercialization and engineering expertise of Bernie Shoor, from a company like BD Biosciences. This partnership enabled the launch of the first-ever commercial FACS flow cytometer—the BD FACS™ II Cell Sorter—by BD Biosciences fifty years ago. The BD FACS™ II Cell Sorter by Becton & Dickinson Immunocytometry Systems (currently BD Biosciences), together with its successors, would unleash scientific discoveries in the field of immunology and medicine in the years to come. Fun fact: the term FACS was trademarked by BD in 1985 and the BD FACS™ II Cell Sorter is currently part of the Smithsonian Institutes collection!
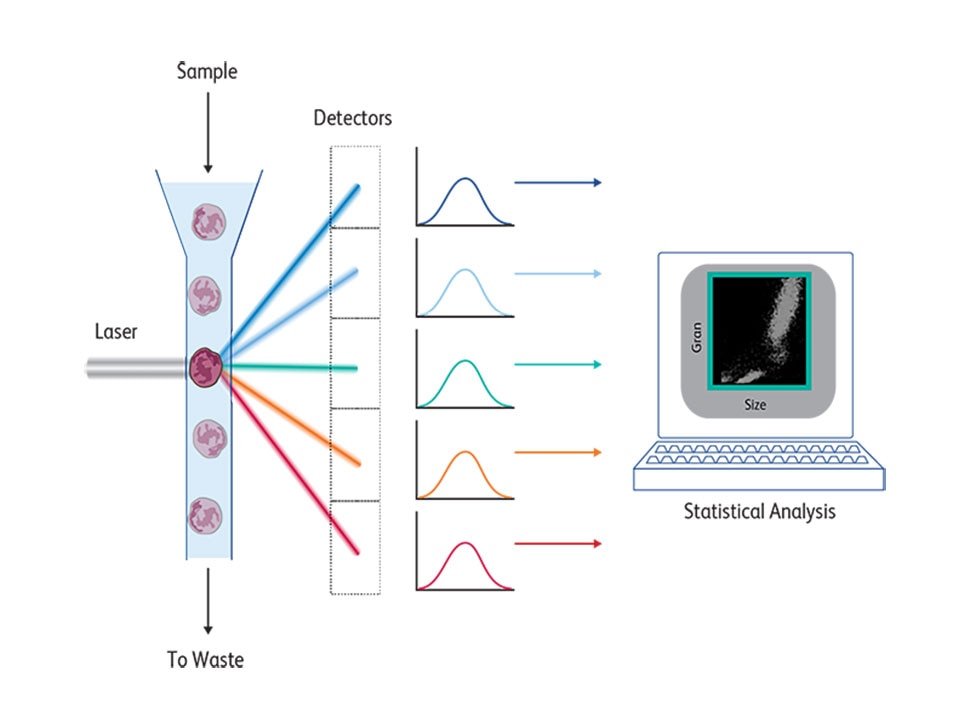
At its core, a FACS instrument measures light emitted by cells stained by fluorescent molecules that are excited by lasers at a certain range and emit at a different range. These emission spectra are collected by detectors and filters with appropriate bandwidths to detect the emission peaks of these fluorochromes. Cells pass one by one across a laser beam for individual analysis following a hydrodynamic focusing based on coaxial laminar flow dynamic properties, described in 1883.4 The first BD FACS™ II Cell Sorter instrument had a laser source that emitted a single wavelength at 488 nm and excited the fluorescein dye. Changing this laser to a krypton laser enabled two-color fluorescence detection using fluoresceine and rhodamine, which was later commercialized by BD as the BD FACS™ IV Flow Cytometer. In the 1980s, krypton lasers were first replaced by helium-neon laser excitation and eventually by solid-state lasers.
As immune cell biology and function were being interrogated more, immunologists began to appreciate the complex nature of immune cells. Leucocytes themselves express numerous proteins and form functionally distinct subpopulations. To identify these various immune subsets and analyze their functional implications, high-parameter analysis was necessary, but to achieve that the technology had to evolve on multiple fronts. Availability of fluorescent dyes was the major limiting factor. Only a few polyclonal antibodies were available and these posed additional challenges due to variability in results from different lots and their cross reactivity. Fortunately, with the introduction of the novel hybridoma technique, a technological breakthrough for producing specific antibodies with antibody-producing B cells fused with immortalized myeloma cells had just been made by Kohler and Milstein.5 The resultant monoclonal antibodies and their high specificity toward specific epitopes of biomarkers and reproducible, reliable detection showed enormous promise for their incorporation in other fields. Herzenberg used the monoclonal antibodies produced using the hybridoma technique in FACS6 and demonstrated the remarkable potential of this combination to open up FACS to become a powerful technique for cell analysis. But for this to be achieved, commercial production of monoclonal antibodies had to happen.
Inception of the BD Monoclonal Center and the evolution of flow cytometry reagents and instrumentation
Continued collaboration between Len Herzenberg of Stanford and Bernie Shoor of BD Biosciences led to the establishment of the BD Monoclonal Center, ensuring a consistent supply of reliable reagents required for FACS-based research. In the early 70s, fluorescein and rhodamine were the only two fluorescent dyes that were available for labeling biomarkers (see timeline of flow cytometry dye evolution). With the introduction of additional laser sources in the UV, violet and blue light range, analysis of markers involved in cell cycle and chromosome analysis became possible. Helium neon lasers enabled effective utilization of algae and cyanobacteria-derived molecules capable of absorbing light energy and transferring to fluorescent molecules, such as phycoerythrin (PE) and allophycocyanin (APC), in FACS.7 The ability to use PE with other dyes opened up a whole new world for higher-parameter flow cytometry analysis of complex cell populations.
In 1985, BD Biosciences introduced the BD FACScan™ Flow Cytometer, the first commercial instrument with the ability to measure three colors simultaneously. This allowed cells to be evaluated under five different dimensions (including two scattering parameters associated to physical characteristics such as size, shape and complexity). In addition, it was the first instrument to introduce fixed alignment with quartz cuvettes, which eliminated the need for daily manual laser adjustments by the instrument operator. The first four-color flow cytometer, the BD FACScan™ Flow Cytometer, underwent its own evolution, from measuring three to up to 10 parameters in the span of a decade, enabling the simultaneous analysis of function and phenotype of immune cell populations, for example, intracellular quantitation of cytokines by specifically identified immune cell subsets.
Along with the addition of a larger number of measurable parameters, another issue that had to be addressed was the sheer size of the flow cytometers and their maintenance. Efforts to miniaturize the setup led to the launch in 1995 of the BD FACSCalibur™ Flow Cytometer, a more compact, benchtop instrument capable of performing analysis and cell sorting, freeing up lab space for researchers. The instrument also included options such as the BD FACS™ Loader and BD® High Throughput Sampler for automation, as well as a specialized software for running various applications.
Introduction of diode red laser lines enabled immunolabeling with cyanin dyes and the development of tandem dyes such as PE-Texas Red, PE-Cy5, PE-Cy5.5 and PE-Cy7.8 An array of these dyes was used to build an 8-color, 10-parameter flow cytometry panel to elucidate leucocyte heterogeneity.9 APC-based tandem dyes and small organic dyes were also commercially introduced in the 1990s, along with semiconductor nanocrystals-based dyes enabling as much as 18-color flow cytometry.8
In addition to now being able to evaluate more surface markers simultaneously, the availability of all these newer fluorochromes had a direct impact on the development of new reagents and assays such as BD Phosflow™ Reagents and bead-based immunoassays called BD® Cytometric Bead Array (CBA). These assays enabled better understanding of cell function with identification of intracellular phosphorylation markers and quantification of secreted cytokines, respectively.
Despite the explosion of all these dye options, the ability of these dyes to effectively absorb light was mostly limited, affecting the sensitivity of assays. This was solved by utilizing the conducting polymer chemistry that was awarded the Nobel Prize in 2000.10 New polymer dyes were developed by Sirigen using a unique class of conducting polymers serving as molecular antennae, which enabled new bright variations of dyes. In 2014, BD Biosciences acquired Sirigen and implemented this technology to develop the BD Horizon Brilliant™ Violet and Ultraviolet Dyes, which changed the landscape of immunophenotyping with their brighter dye options. 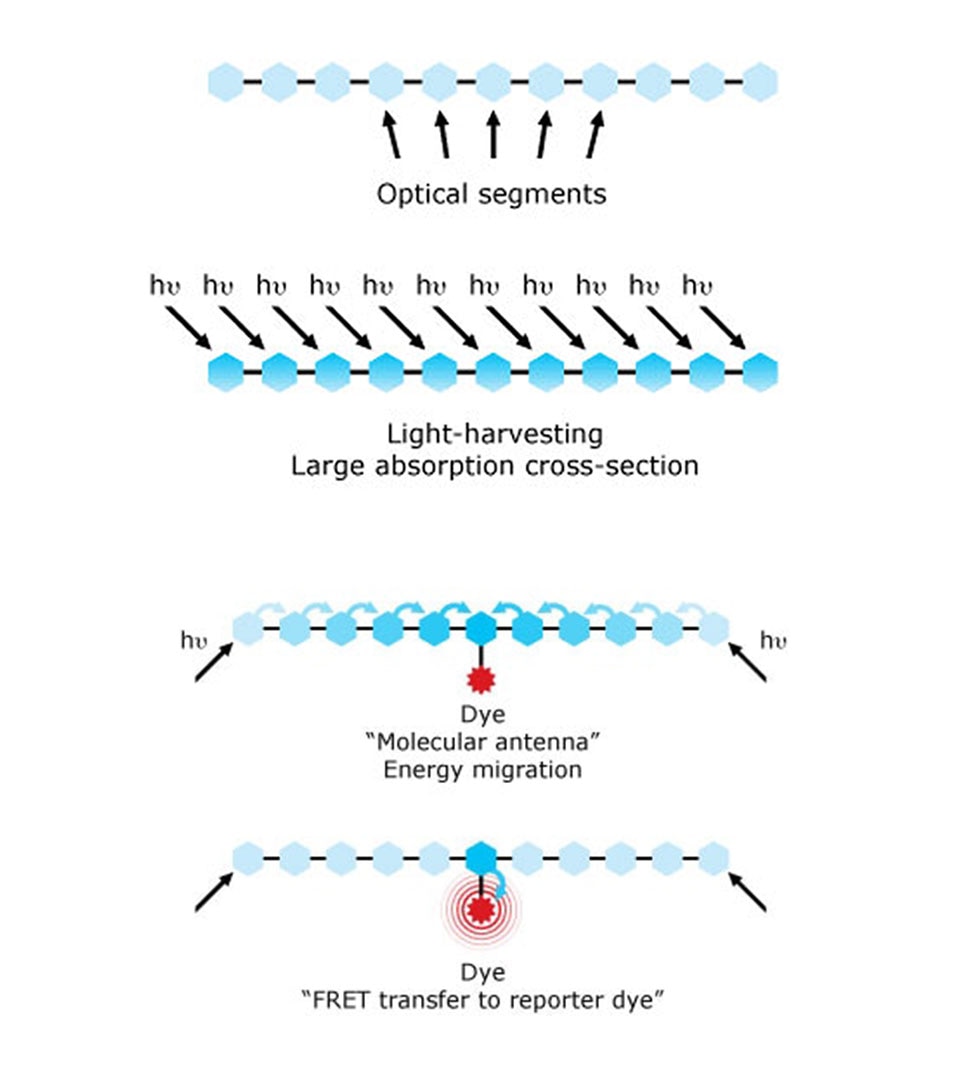
In parallel, instrumentation capabilities were advancing at a rapid pace. In the first decade of the millennium, BD introduced several cell analyzers and sorters. Fun fact, one of those instruments, the BD FACSVantage™ Flow Cytometer, has been moved to Massachusetts General Hospital’s Paul S. Russell, MD Museum of Medical History and Innovation for its use in cutting-edge research on organ transplantation. The first 7-laser special-order (configured to suit the users’ specific research needs) BD® LSR II Flow Cytometer in 2008 has contributed to many scientific discoveries. The introduction of another custom instrument, the BD LSRFortessa™ X-20 Cell Analyzer, which was compatible with BD Horizon Brilliant™ Violet Dyes and BD FACSDiva™ Software, enabled the analysis of 20 parameters simultaneously. In addition to higher-parameter cell analyzers, BD also introduced simplified benchtop cell sorters, such as the BD FACSAria™ Cell Sorter. This was the first technology to combine electromagnet droplet sorting with fixed alignment lasers to a gel coupled cuvette flow cell, delivering performance and experience similar to those of a cell analyzer while also introducing transformative automation to the sorting workflow with BD FACS™ Accudrop and Sweet Spot Technologies, making cell sorting more accessible to researchers.
In the 2010s, innovation continued to push the evolution of instruments in BD Biosciences. By implementing automated processes, benchtop sorters extended the capability of cell sorting, while still allowing it to be easily accessible to researchers. This accomplishment led the way to the development of smaller and more automated instruments overall, including the BD FACSVerse™ Flow Cytometer and BD FACSMelody™ Cell Sorter.
At the same time, the importance of standardization of methods, elimination of manual processes and workflow inconsistencies between and within labs, which are critical for clinical labs where reproducibility and reliability are of paramount importance, catalyzed the evolution of clinical flow cytometers with these capabilities. The introduction of automation supporting more standardized processes drove the need for next-generation clinical flow cytometers, such as the BD FACSLyric™ Flow Cytometer, integrated with the FACSDuet™ Premium Sample Preparation System, which delivers the workflow efficiencies and results required for clinical labs. The paired evolution of analyzers and sorters, aligned with the development of newer fluorochrome options excited out of the UV laser, the BD Horizon Brilliant™ Ultraviolet Dyes, also resulted in the introduction of a new family of high-parameter instruments to support multicolor experiments in various fields including immunology and immune-oncology. The BD FACSymphony™ A3 Cell Analyzer and BD FACSymphony™ A5 Cell Analyzer and the BD FACSymphony™ S6 Cell Sorter provided the ability to generate 30-parameter flow cytometry data and beyond using the conventional approach of using individual PMT detectors filtered to evaluate specific fluorochromes and applying traditional compensation workflows.
Evolution of flow cytometry software
Another rate-limiting factor in the evolution of flow cytometry was the need for smarter software that enabled the analysis of all multiparametric flow cytometry data. Conventional flow cytometry data analysis entails manual gating of areas and the need to analyze combinations of different parameters. In the beginning when only a few parameters were measured, this was rather straightforward and could be done using simple two-dimensional plots. But as the number of fluorochromes and consequently the number of parameters that could be measured increased, the number of two-dimensional plots increased exponentially, making data analysis extremely complicated. The limitations of existing software hindered expansion of the number of parameters that could be measured for the first 15 years after the introduction of FACS in the 1970s.
In general, flow cytometers are equipped with instrument-specific software, such as the BD FACSDiva™ Software introduced in 2002, which supports ten BD flow cytometers currently! In addition to software embedded in the instruments, during the 1990s, standalone software, such as the FlowJo™ Software, were introduced. This software provided analysis capabilities and options that could add flexibility and power to flow cytometry data analysis, thereby supporting its continuous advancements towards delivering more data parameters and allowing more analytical dimensions of each cell. Throughout the years, FlowJo™ Software continuously evolved following the same rhythm of evolution of the instruments. With nearly three decades and 10 main versions, the instrument-agnostic FlowJo™ Software has adapted to support the constant changes of files produced by the newer generations of flow cytometers while providing newer ways to analyze, interpret, visualize and display data. Currently, this software is at the forefront of incorporating dimensionality reduction approaches such as tSNE and UMAP to the flow cytometry field as a means of supporting the higher-parameter data introduced by the latest technologies allowing researchers to find their answers faster than before.
The advent of spectral flow cytometry
A different method of cell analysis using spectral flow cytometry has been gaining momentum in the past decades. Spectral flow cytometry technology was first introduced in 2004 at the congress of the International Society for Analytical Cytology,11 and has since evolved. As opposed to conventional flow cytometers that collect only a discrete portion of the emission spectrum as defined by a single filter and detector per fluorochrome, spectral flow cytometers collect the entire visible spectrum with minimal gaps. The emission spectrum of each fluorochrome is collected by an array of filtered detectors. A spectral unmixing algorithm is used to determine the contribution of individual fluorochromes, distinguishing it from the traditional algorithms used for compensation on conventional flow cytometers. The spectral-enabled BD FACSymphony™ A5 SE Cell Analyzer, introduced by BD in 2021, offers the ability to gain full visible spectrum coverage and allows over 40 color measurements (up to 50 parameters), enabling both conventional and spectral flow cytometry. The simultaneous development of spectrally enabled fluorochromes, such as the BD Horizon RealYellow™ and BD Horizon RealBlue™ Dyes, which are engineered to reduce spillover, has elevated the power of cell analysis using spectral flow cytometry. In 2023, this evolution resulted in the introduction of the newest technology from BD, for spectral analysis. BD SpectralFX™ Technology, present in the BD FACSDiscover™ S8 Cell Sorter, enables full spectrum cell analysis and sorting through 78 fluorescence APD detectors paired with algorithmically optimized filter bandwidths.
Imaging cytometry
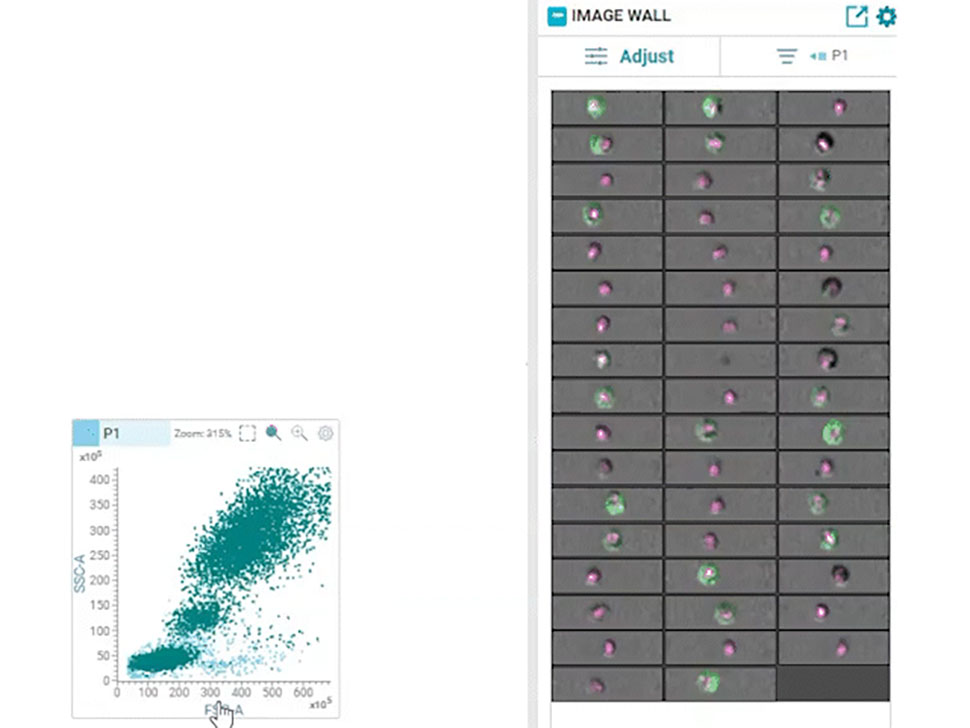
Multiparametric data analysis can yield important insights based on the intensity of fluorescence signals within gated regions on two-dimensional contour plots. However, this approach lacks the spatial resolution that can be obtained from imaging single cells and is prone to yielding erroneous results with the inability to distinguish between doublets, debris and actual single cells. In addition, intracellular localization of protein biomarkers could not be easily measured with this technique. Imaging cytometry addressed some of these issues; It combined flow cytometry with imaging and enabled imaging of single cells, but it could not be combined with high-speed sorting. The combination of real-time imaging of cells and high-speed, high-throughput sorting had remained an unfulfilled dream for several decades.
In 2022, BD Biosciences brought this dream to reality by launching the groundbreaking BD CellView™ Image Technology that enables ultrafast imaging of cells together with high-speed cell sorting. It utilizes technology from the wireless communication industry to obtain multicolor fluorescent images in real-time and uses that information to sort cells at a speed of up to 15,000 events per second!12
In 2023, this technology was combined with BD SpectralFX™ Technology, which enables full spectrum cell sorting, to create a first-of-its-kind real-time imaging spectral cell sorter: the BD FACSDiscover™ S8 Cell Sorter. This combination of spectral flow cytometry with real-time spatial and morphological insights (RTI-SFC) , along with new modular optical architecture and system-aware algorithms, has opened the door for limitless possibilities for scientists.
From the time of introduction of FACS in the 1970s to the five decades that have followed, the incredible power of its use in multiparametric high-dimensional analysis for discerning, enumerating and sorting immune cell subpopulations has been realized and its utilization in clinical settings has become apparent. One of the first fields to benefit from it was HIV research. In the 1980s, at the peak of the AIDS outbreak, FACS was incorporated into clinical diagnostics and subsequently for HIV evaluation and monitoring. Flow cytometry was instrumental in establishing that the depletion of CD4+ cells was the signature manifestation of HIV infection.13 Three-color flow cytometry to measure CD4+ helper and CD8+ cytotoxic cells facilitated the clinical utility of this technique. The incredible volume of subsequent research utilized flow cytometry for identifying markers of activated T cells, early T cells, regulatory T cells and of T-cell function.14 It was no surprise that clinical flow cytometry was first used in the management of HIV patients. In addition to HIV, research on leukemia and lymphoma also benefited enormously from the use of flow cytometry in the years that followed. The potential use of small particles such as extracellular vesicles as biomarkers has been investigated extensively in recent years, and advancements in instrumentation for small particle detection, such as that provided by the BD FACSymphony™ A1 Cell Analyzer, is helping scientists push the boundaries of research in this field.15,16
In the past few decades, the burgeoning field of cancer immunotherapy went from bench to bedside, with rapid progress arising from the former with respect to unraveling the biology behind immune checkpoint inhibition. A new paradigm was established in cancer therapy using the approach of targeting inhibitory pathways that blocked an effective immune response against cancer. Flow cytometry played a major part in elucidating the role of cytotoxic T-lymphocyte associated antigen 4 (CTLA-4) protein and the immunoinhibitory receptor PD-1 blockade to enhance anti-tumor immunity .17,18,19 Both James Allison and Tasuku Honjo, the scientific brains behind these strategies, received the Nobel Prize in Physiology and Medicine in 2018. BD Biosciences was proud to be part of their journey, with the use of several BD flow cytometers, such as the BD FACScan™, BD® LSR II and BD FACSCalibur™, in their landmark experiments.
With all this progress, we are only just beginning to unravel what is possible using flow cytometry. The combination of real-time imaging with spectral flow cytometry on the FACSDiscover™ S8 Cell Sorter has opened up a world of possibilities for what can be measured. The future of flow cytometry remains boundless.
