- United States (English)
- United States (English)
- United States (English)
- United States (English)
- United States (English)
- United States (English)
- United States (English)
- United States (English)
- United States (English)
- United States (English)
- United States (English)
- United States (English)
- United States (English)
- United States (English)
- United States (English)
-
Your selected country is
United States
- Change country/language
-
Promotions
-
sk-testpage
-
BD Primer Program
-
BD Discovery 2021
-
Reagents
-
DO NOT PUBLISH
-
Reagentes Com Valor Promocional
-
Lunch Box Giveaway
-
EQCプログラム 外部精度管理 施設間精度管理
-
Backbone Reagents Promo
-
Backbone Reagents Promo
-
Classic Dyes
-
Back to Lab
-
End of Year
-
Tcell Backbone Panel Promotion
-
BD Horizon™ Human T Cell Backbone Panel
-
New Lab Promotion
-
Flash Sale
-
BD Panel Design Program
-
Real Dyes Sample Offer
-
BD’s 50 Years of Innovation Research Instrument Promotion
-
BD FACSLyric™ Flow Cytometers 50th Anniversary Promo
-
BD FACSAria™ Customer Loyalty Promotion
-
FlowJo™ Software Promotion
-
BD® Research Cloud Promotion
-
sk-testpage
-
Custom BD® AbSeq
-
Custom AbSeq FAQ Answer 2
-
Custom AbSeq FAQ Answer 3
-
Custom AbSeq FAQ Answer 4
-
Custom AbSeq FAQ Answer 5
-
Custom AbSeq FAQ Answer 6
-
Custom AbSeq FAQ Answer 7
-
Custom AbSeq FAQ Answer 8
-
Custom AbSeq FAQ Answer 9
-
Custom AbSeq FAQ Answer 10
-
Custom AbSeq FAQ Answer 11
-
Custom AbSeq FAQ Answer 12
-
Custom AbSeq FAQ Answer 13
-
Custom AbSeq FAQ Answer 14
-
Custom AbSeq FAQ Answer 15
-
Custom AbSeq FAQ Answer 16
-
Custom AbSeq FAQ Answer 17
-
Custom AbSeq FAQ Answer 18
-
Custom AbSeq FAQ Answer 19
-
Custom AbSeq FAQ Answer 20
-
Custom AbSeq FAQ Answer 2
- United States (English)
- United States (English)
- United States (English)
- United States (English)
- United States (English)
- United States (English)
- United States (English)
- United States (English)
- United States (English)
- United States (English)
- United States (English)
- United States (English)
- United States (English)
- United States (English)
- United States (English)
-
Change country/language
Old Browser
Your account has been put on hold due to inactivity. To re-activate, check your account information and make all necessary updates.
Looks like you're visiting us from United States.
Would you like to stay on the current country site or be switched to your country?
Your account has been put on hold due to inactivity. To re-activate, check your account information and make all necessary updates.
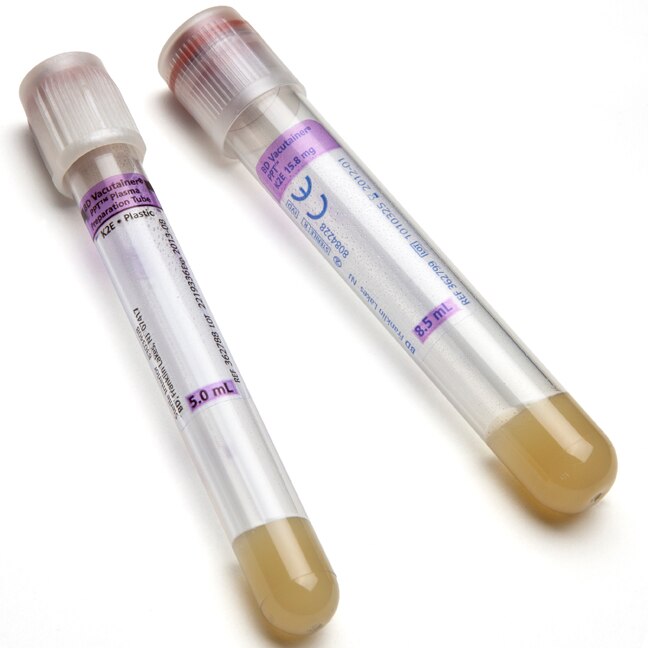
BD Vacutainer® PPT™ Plasma Preparation Tube - Paper Label

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Product Details
Description
BD Vacutainer® PPT™ is a closed system allowing separation and storage of undiluted EDTA plasma in the primary blood collection tube. It is intended for the purposes of molecular diagnostic testing.
PPT™ is an evacuated, sterile blood collection tube that contains an inert gel and spray-dried K2EDTA anticoagulant for achieving plasma separation. When the sample-filled tube is subjected to centrifugation, the gel migrates and forms a physical barrier between the plasma and most of the cellular elements. Since the tube uses spray-dried K2EDTA as opposed to liquid additives, the plasma obtained is undiluted.
| Tube Material | Plastic |
| Tube Size | 16x100 mm |
| Draw Volume | 8.5 mL |
| Closure Type | BD Hemogard™ |
| Closure Color | Pearl White |
| Label | Paper |
| Cell Separator | Yes (Gel) |
| Anticoagulant | K2EDTA |
| Sterilization | Radiation , 10-6 SAL , ISO11137-1 |
- BD White Paper VS8188: Evaluation Of The Effect Of Specimen Handling Conditions In BD Vacutainer® PPT On The Stability Of HIV-1 Viral Load Using Roche Cobas® Ampliprep/ Cobas® Taqman® HIV-1 Test, 2010.
- BD White Paper VS8189: Evaluation of Specimen Handling Conditions in BD Vacutainer® Plasma Preparation Tube HIV-1 Viral Load as Measured by the Abbott RealTime HIV-1 Assay.
- Evaluation of the VACUTAINER® Brand PPT™ Tubes for HCV Viral Load Testing.
- Determination of the Effect of Freezing BD Vacutainer® PPT™ Plasma in situ on Hepatitis C (HCV) Viral Loads as Measured by the Roche COBAS® TaqMan® HCV ASR.
- Determination of the Effect of Freezing BD Vacutainer® PPT™Plasma in situ on Hepatitis B (HBV) Viral Loads Using the Roche COBAS® TaqMan® HBV RUO Assay.
Preparation And Storage
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.