-
抗体試薬
- フローサイトメトリー用試薬
-
ウェスタンブロッティング抗体試薬
- イムノアッセイ試薬
-
シングルセル試薬
- Accessories
- BD® AbSeq Assay | シングルセル試薬
- BD Rhapsody™ Accessory Kits | シングルセル試薬
- BD® Single-Cell Multiplexing Kit | シングルセル試薬
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits | シングルセル試薬
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit | シングルセル試薬
- BD® OMICS-Guard Sample Preservation Buffer
- BD Rhapsody™ ATAC-Seq Assays
-
細胞機能評価のための試薬
-
顕微鏡・イメージング用試薬
-
細胞調製・分離試薬
- Dehydrated Culture Media
-
- Accessories
- BD® AbSeq Assay | シングルセル試薬
- BD Rhapsody™ Accessory Kits | シングルセル試薬
- BD® Single-Cell Multiplexing Kit | シングルセル試薬
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits | シングルセル試薬
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit | シングルセル試薬
- BD® OMICS-Guard Sample Preservation Buffer
- BD Rhapsody™ ATAC-Seq Assays
- Japan (Japanese)
-
Change country/language
Old Browser
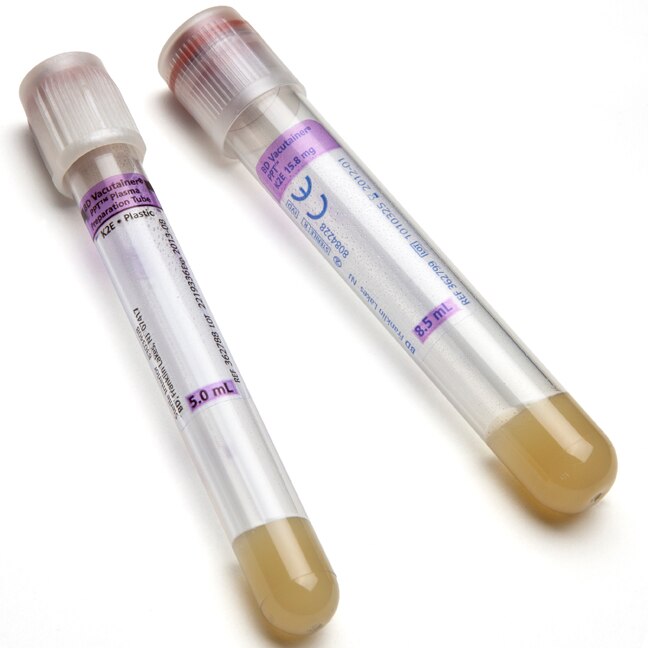
BD Vacutainer® PPT™ Plasma Preparation Tube - Paper Label

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Product Details
Description
BD Vacutainer® PPT™ is a closed system allowing separation and storage of undiluted EDTA plasma in the primary blood collection tube. It is intended for the purposes of molecular diagnostic testing.
PPT™ is an evacuated, sterile blood collection tube that contains an inert gel and spray-dried K2EDTA anticoagulant for achieving plasma separation. When the sample-filled tube is subjected to centrifugation, the gel migrates and forms a physical barrier between the plasma and most of the cellular elements. Since the tube uses spray-dried K2EDTA as opposed to liquid additives, the plasma obtained is undiluted.
| Tube Material | Plastic |
| Tube Size | 16x100 mm |
| Draw Volume | 8.5 mL |
| Closure Type | BD Hemogard™ |
| Closure Color | Pearl White |
| Label | Paper |
| Cell Separator | Yes (Gel) |
| Anticoagulant | K2EDTA |
| Sterilization | Radiation , 10-6 SAL , ISO11137-1 |
- BD White Paper VS8188: Evaluation Of The Effect Of Specimen Handling Conditions In BD Vacutainer® PPT On The Stability Of HIV-1 Viral Load Using Roche Cobas® Ampliprep/ Cobas® Taqman® HIV-1 Test, 2010.
- BD White Paper VS8189: Evaluation of Specimen Handling Conditions in BD Vacutainer® Plasma Preparation Tube HIV-1 Viral Load as Measured by the Abbott RealTime HIV-1 Assay.
- Evaluation of the VACUTAINER® Brand PPT™ Tubes for HCV Viral Load Testing.
- Determination of the Effect of Freezing BD Vacutainer® PPT™ Plasma in situ on Hepatitis C (HCV) Viral Loads as Measured by the Roche COBAS® TaqMan® HCV ASR.
- Determination of the Effect of Freezing BD Vacutainer® PPT™Plasma in situ on Hepatitis B (HBV) Viral Loads Using the Roche COBAS® TaqMan® HBV RUO Assay.
Preparation And Storage
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.